Interface Options Available for This Product:
- While there are no interface options currently available, we are always working to improve our interface design based on feedback from our current clients. Check back for updates!
Who Does BioTrax QMS Help?
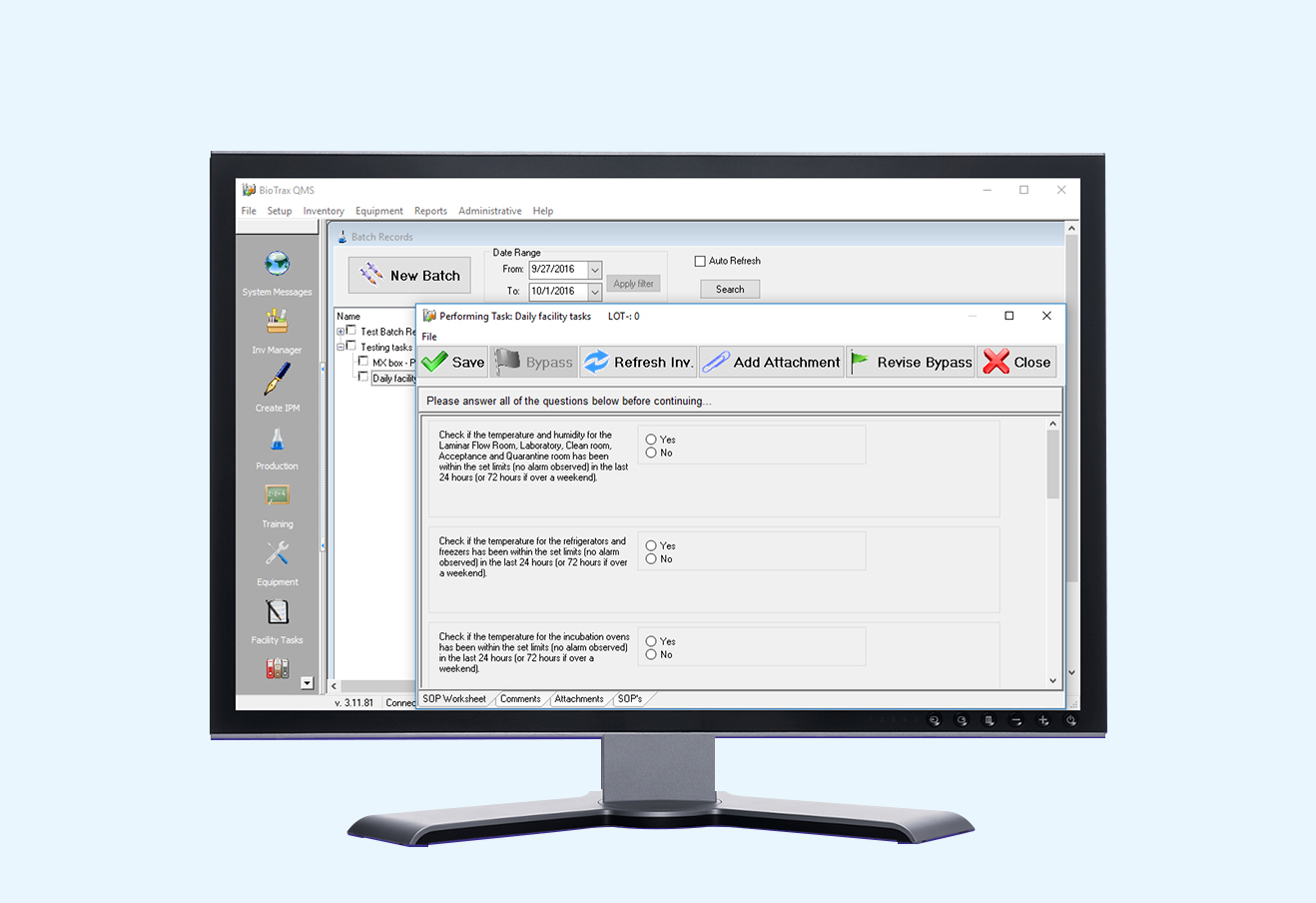
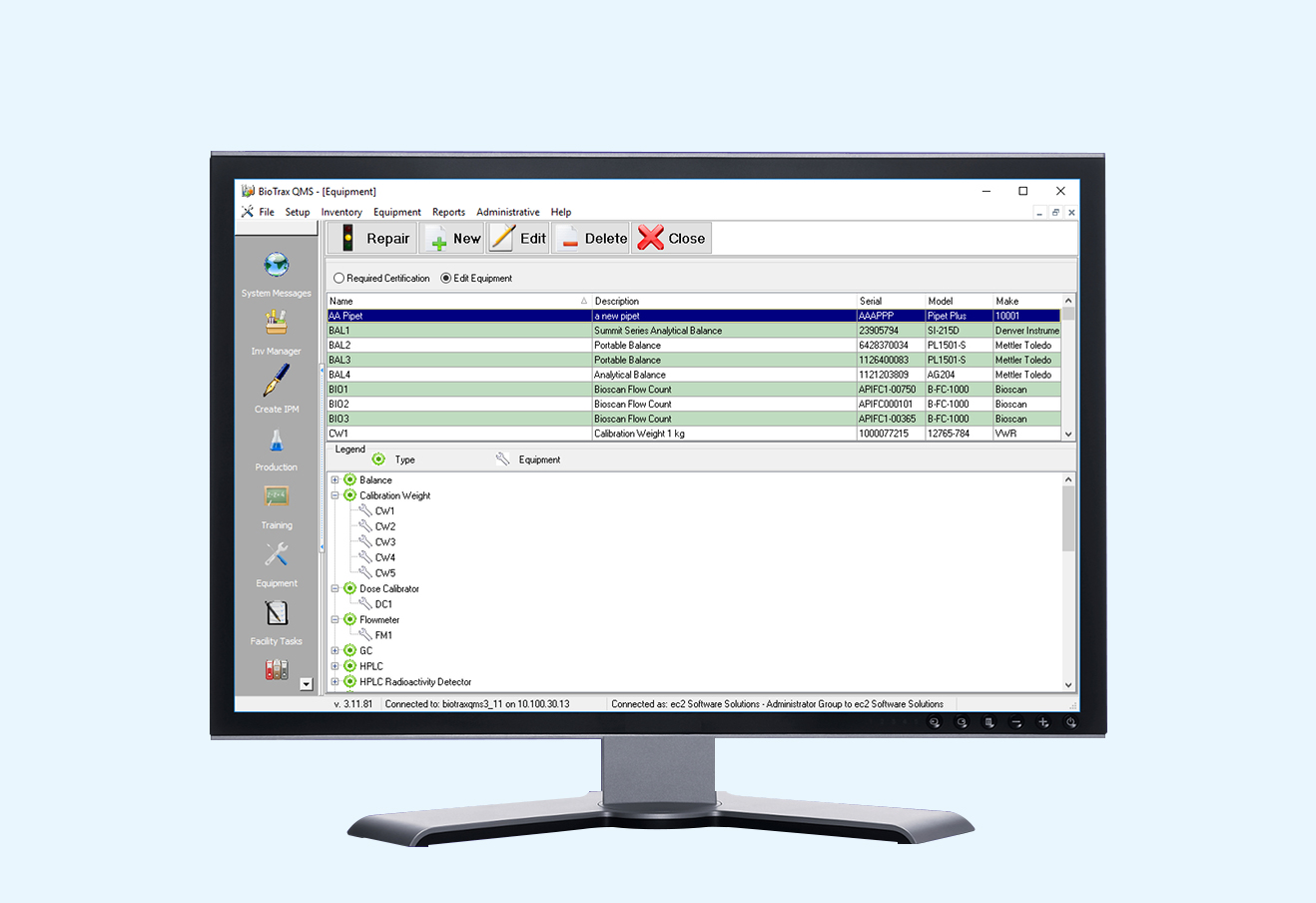
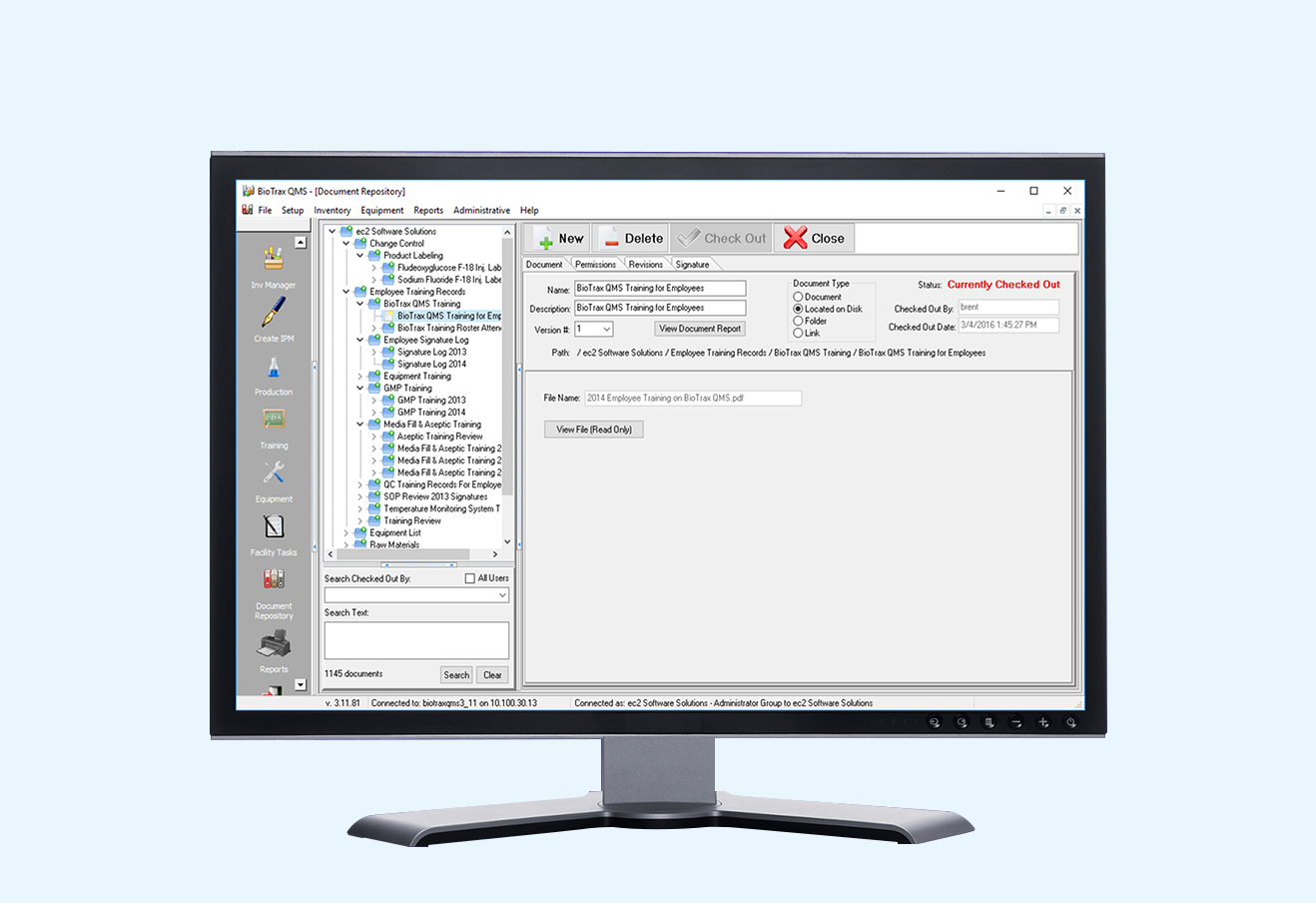
BioTrax QMS was created to provide your facility with an easily accessible, comprehensive database and an efficient system for inventory tracking and batch record maintenance. If you are one of these people, BioTrax QMS will make your life easier:

Pharmacist

Cyclotron Engineer

Administration

Radiochemist

Technician

Production Management
BioTrax QMS Customers

"The inventory capabilities (raw material check-in, IPM batches, etc.) of BioTraxQMS are superb, and allow for a seamless incorporation into the respective batch records."

“I have nothing but good things to say about BioTrax QMS."